Digital PCR Technology: A New Standard for Precision in Molecular Diagnostics
Digital PCR Technology: A New Standard for Precision in Molecular Diagnostics
Executive Summary
Digital PCR (dPCR) represents a revolutionary advancement in molecular diagnostics, offering absolute quantification of nucleic acids without the need for standard curves. This technology partitions PCR reactions into thousands to millions of separate compartments, enabling unprecedented sensitivity and precision in detecting rare targets. Recent innovations in chipless systems, AI-driven analysis, and multiplex capabilities are expanding its clinical utility while addressing traditional limitations in cost and workflow complexity.
Table of Contents
- Introduction and Principles
- Technical Advantages Over Conventional PCR
- Current Technological Platforms
- Key Applications
- Recent Advances and Innovations (2020-2025)
- Current Limitations and Challenges
- Future Directions and Emerging Trends
- Conclusions
- References
Introduction and Principles
Fundamental Concept
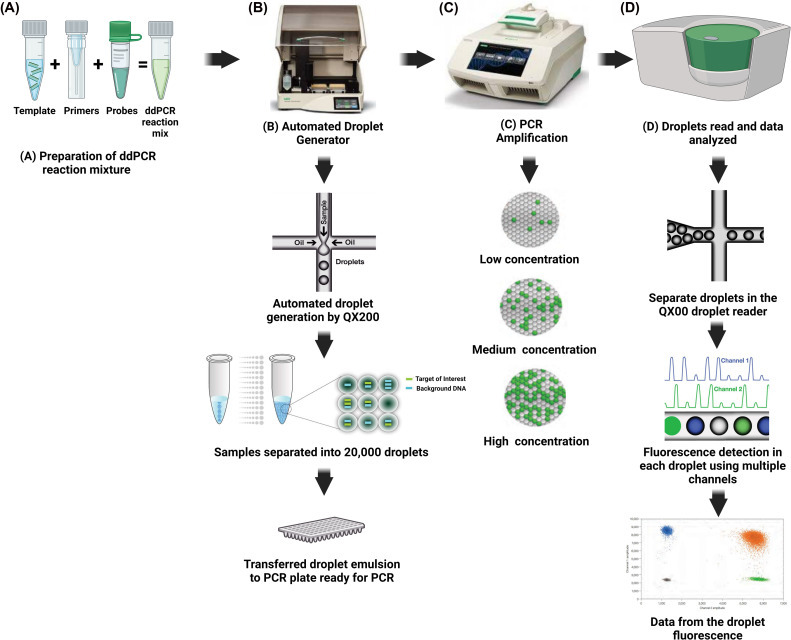
Digital PCR partitions a PCR mixture into thousands to millions of separate reactions, ensuring that each partition contains zero or one target molecule [1]. Following amplification, end-point positive/negative calls combined with Poisson statistics yield absolute concentration measurements without requiring external calibrators or standard curves.
Core Methodology
The dPCR workflow involves three essential steps:
- Sample partitioning into numerous independent reactions
- PCR amplification within each partition
- Binary detection and statistical analysis to determine absolute target concentration
This partition-and-count approach fundamentally differs from quantitative PCR (qPCR), which relies on amplification kinetics and requires standard curves for quantification [2].
Technical Advantages Over Conventional PCR
1. Absolute Quantification
Unlike qPCR, dPCR provides absolute quantification without external calibrators or standard curves. Concentration is directly inferred from the fraction of positive partitions, eliminating calibration-related errors and improving reproducibility across laboratories [1].
2. Superior Sensitivity for Rare Targets
Digital PCR excels at detecting and quantifying targets present at extremely low fractional abundance. The technology can discriminate rare mutations and detect small copy-number variations with sensitivities reaching 1 in 100,000 wild-type backgrounds in optimized workflows [3].
3. Enhanced Inhibitor Tolerance
Partitioning reduces bulk inhibitory effects by isolating reactions, improving quantitation in complex matrices including clinical samples, environmental specimens, and food products. This resilience makes dPCR particularly valuable for challenging sample types [2,3].
4. Improved Reproducibility
End-point detection and binary readouts reduce dependence on amplification kinetics and minimize instrument-to-instrument variability. This results in higher reproducibility compared to qPCR, especially for low-abundance targets [2].
5. Precision at Low Copy Numbers
Digital PCR demonstrates superior precision when quantifying low-abundance targets, where Poisson noise dominates in conventional PCR methods. This advantage is particularly pronounced for copy numbers below 100 per reaction [3].
Current Technological Platforms
Droplet Digital PCR (ddPCR)
Commercial Systems: Bio-Rad QX200 and QX ONE systems represent the most widely adopted dPCR platforms.
Key Features:
- Oil-emulsified microdroplets produced by automated instruments
- High droplet counts per run (up to 20,000 droplets per well)
- Established two- to four-color assays for multiplexing
- Supported by comprehensive commercial workflows and quality control protocols [3,5]
Applications: Widely used for liquid biopsy, viral load monitoring, and copy number variation analysis.
Chip-Based Systems
Architecture: Fixed microchamber arrays on silicon chips or polymer substrates.
Advantages:
- Precise partition volume control
- High partition density
- Compatible with automated liquid handling
Limitations: Higher consumable costs and potential for chip-to-chip variability.
Novel Platforms
OsciDrop System
A revolutionary chipless approach using pipette-based droplet printing without microfabricated chips [6].
Features:
- Automated end-to-end workflow
- Demonstrated quadruplex assays
- Digital stepwise melting analysis (dSMA) profiling
- Machine learning-driven image analysis
- Reduced consumable costs compared to chip systems
Microwell Arrays
Characteristics:
- Fixed partition format in microwell plates
- Simplified instrumentation requirements
- Lower throughput but reduced complexity
Key Applications
1. Clinical Diagnostics
Liquid Biopsy
- Circulating tumor DNA (ctDNA) detection and quantification
- Minimal residual disease monitoring in cancer patients
- Mutation detection at fractional abundances below qPCR detection limits
Infectious Disease Monitoring
- Viral load quantification with superior precision at low copy numbers
- Antimicrobial resistance gene detection
- Pathogen identification in complex clinical matrices [4]
Prenatal Diagnostics
- Copy number variation detection in cell-free DNA
- Aneuploidy screening with improved accuracy
- Rare fetal cell analysis
2. Research Applications
Genomics Research
- Copy number variation studies
- Single-cell analysis applications
- Epigenetic modifications detection through methylation-specific assays
Environmental Monitoring
- Microbial biodegradation monitoring [11]
- Pathogen detection in water systems
- GMO detection and quantification in food products [12]
Agricultural Biotechnology
- Transgene copy number determination
- Seed purity testing
- Food authenticity verification
3. Pharmaceutical Development
Drug Development
- Pharmacokinetic studies with enhanced precision
- Biomarker quantification for therapeutic monitoring
- Cell line characterization and quality control
Recent Advances and Innovations (2020-2025)
1. Chipless Droplet Generation Technologies
The OsciDrop system represents a breakthrough in reducing consumable costs while maintaining high performance [6]. This pipette-based approach eliminates expensive microfluidic chips while enabling:
- Automated droplet generation and processing
- High-multiplex capabilities through digital stepwise melting analysis
- Machine learning-enhanced image analysis for improved accuracy
2. Poisson-Independent Quantification Methods
Competitive microdroplet PCR offers an alternative to traditional Poisson-based quantification [7]. This approach:
- Uses internal competitor DNA for quantification via equivalence point analysis
- Reduces misclassification errors
- Avoids Poisson distribution assumptions
- Shortens analysis time and reduces post-PCR processing steps
3. Artificial Intelligence Integration
AI and machine learning are revolutionizing dPCR analysis through [5,10]:
- Deep learning algorithms for partition classification with >96% accuracy
- Automated quality control and data validation
- Single-channel multiplexing strategies via kinetic and thermodynamic feature analysis
- Real-time optimization of assay parameters
4. Enhanced Multiplexing Capabilities
Recent developments in multiplexing include:
- Seven-target EGFR variant profiling using digital stepwise melting analysis [6]
- Standardized frameworks for non-competing multiplex assay design [8]
- Improved probe design strategies for cluster separation
5. Label-Free Detection Methods
Flow-LAMP technology demonstrates label-free digital nucleic acid amplification testing [14]:
- Uses scatter-based detection via flow cytometry
- Achieves limit of detection ~38.15 copies/µL
- Eliminates fluorescence requirements
- Reduces instrumentation complexity
6. Clinical Translation Advances
Single-Cell Digital PCR
Development of methods for HBV DNA quantification in liver biopsy tissues at the single-cell level [13], enabling:
- Enhanced sensitivity for viral detection
- Improved understanding of infection dynamics
- Better therapeutic monitoring capabilities
Methylation-Specific Applications
Droplet digital methylation-specific PCR for gastric cancer diagnostics [15]:
- DNA-based cytology applications
- Enhanced clinical utility for cancer detection
- Improved diagnostic accuracy
Current Limitations and Challenges
1. Cost and Economic Barriers
High Infrastructure Costs:
- Expensive instrumentation ($100,000-$300,000 for commercial systems)
- Costly consumables limiting routine clinical deployment
- Particularly challenging for low- and middle-income countries [5]
2. Multiplexing Constraints
Technical Limitations:
- Practical multiplexing often limited by available fluorescent channels
- Complex assay design requirements for non-competing targets
- Cluster separation challenges in high-multiplex assays [8]
3. Standardization Issues
Regulatory and Quality Control Gaps:
- Limited standardized protocols for method verification [12]
- Inter-laboratory comparability challenges
- Need for harmonized quality control guidelines
- Domain-specific validation frameworks still developing
4. Workflow Complexity
Multi-Step Process Requirements:
- Sample preparation complexity
- Multiple instrumentation requirements
- Manual intervention points
- Limited fully integrated sample-to-answer systems [6,5]
5. Technical Challenges
Partition Classification Errors:
- Misclassification of ambiguous partitions can bias results
- Rain and intermediate fluorescence populations
- Need for improved algorithms and quality metrics [7,5]
Future Directions and Emerging Trends
1. Artificial Intelligence and Automation
Expected Developments:
- Broader adoption of machine learning for partition classification
- Automated quality control workflows to reduce operator variability
- AI-driven assay optimization and design
- Real-time adaptive analysis algorithms [5]
2. Cost Reduction Strategies
Technological Solutions:
- Chipless droplet generation systems (e.g., OsciDrop) [6]
- Label-free detection methods (e.g., Flow-LAMP) [14]
- Simplified instrumentation designs
- Reduced consumable costs through manufacturing innovations
3. Alternative Quantification Paradigms
Non-Poisson Methods:
- Competitive/ratio-based quantification approaches [7]
- Improved accuracy in variable partitioning systems
- Reduced sensitivity to partition volume variations
- Enhanced robustness for challenging samples
4. Clinical Translation Acceleration
Precision Medicine Applications:
- High-sensitivity ctDNA assays for therapeutic monitoring
- Methylation-specific assays for micrometastatic detection [15]
- Single-cell digital PCR for rare cell analysis [13]
- Personalized therapeutic monitoring protocols
5. Regulatory Framework Development
Standardization Initiatives:
- Comprehensive method verification guidelines [12]
- Inter-laboratory comparison protocols
- Clinical validation frameworks
- Accreditation standards for diagnostic laboratories
6. Point-of-Care Applications
Decentralized Testing:
- Portable dPCR systems for field applications
- Simplified sample preparation protocols
- Rapid turnaround time systems
- Integration with telemedicine platforms
7. Enhanced Multiplexing
Next-Generation Capabilities:
- Higher-order multiplexing through advanced probe chemistries
- Spectral unmixing technologies
- Time-resolved detection methods
- Combinatorial encoding strategies
Conclusions
Digital PCR has emerged as a transformative technology in molecular diagnostics, offering unparalleled precision and sensitivity for nucleic acid quantification. The technology's ability to provide absolute quantification without standard curves, combined with superior performance for rare target detection, positions it as an essential tool for precision medicine and advanced research applications.
Recent innovations in chipless systems, AI-driven analysis, and alternative quantification methods are addressing traditional limitations while expanding the technology's accessibility and utility. The integration of artificial intelligence is particularly promising, offering solutions for automated analysis, quality control, and assay optimization.
However, significant challenges remain, including high costs, workflow complexity, and the need for standardized protocols. The development of cost-effective platforms, simplified workflows, and comprehensive regulatory frameworks will be crucial for broader adoption, particularly in routine clinical settings and resource-limited environments.
Looking forward, digital PCR is poised for continued growth and innovation. The convergence of technological advances, regulatory developments, and clinical validation efforts suggests that dPCR will play an increasingly important role in personalized medicine, infectious disease management, and precision diagnostics. The technology's unique capabilities make it particularly well-suited for emerging applications in liquid biopsy, single-cell analysis, and point-of-care diagnostics.
As the field continues to mature, the focus on standardization, cost reduction, and workflow simplification will be essential for realizing the full potential of digital PCR technology in improving patient care and advancing scientific research.
References
[1] Digital PCR: from early developments to its future application in clinics. Lab on a Chip. 2025. DOI: 10.1039/d5lc00055f
[2] Li, Z. The Principle, Application and Development Prospect of the Technology PCR. Theoretical and Natural Science. 2025. DOI: 10.54254/2753-8818/2025.au23543
[3] de Korne-Elenbaas, J., Caduff, L., Lison, A., et al. Design, validation and implementation of multiplex digital PCR assays for simultaneous quantification of multiple targets. Letters in Applied Microbiology. 2024. DOI: 10.1093/lambio/ovae137
[4] Mirabile, A., Sangiorgio, G., Bonacci, P.G., et al. Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings. Diagnostics. 2024. DOI: 10.3390/diagnostics14151598
[5] Waziri, I., Usman, M., Dandalma, Z.A. PyPCRtool: A python package for in silico PCR and primer verification. Dutse Journal of Pure and Applied Sciences. 2024. DOI: 10.4314/dujopas.v10i3a.25
[6] Li, C., Kang, N., Ye, S., et al. All-In-One OsciDrop Digital PCR System for Automated and Highly Multiplexed Molecular Diagnostics. Advanced Science. 2024. DOI: 10.1002/advs.202309557
[7] Ganguly, R., Lee, C.S. A Poisson-Independent Approach to Precision Nucleic Acid Quantification in Microdroplets. ACS Applied Bio Materials. 2024. DOI: 10.1021/acsabm.4c00350
[8] Sancha Dominguez, L., Cotos Suárez, A., Sánchez Ledesma, M., et al. Present and Future Applications of Digital PCR in Infectious Diseases Diagnosis. Diagnostics. 2024. DOI: 10.3390/diagnostics14090931
[9] NUS iGEM. Overlap PCR v1. 2023. DOI: 10.17504/protocols.io.bp2l6x8zrlqe/v1
[10] Zhang, Y., Liu, X., Xu, C., et al. Artificial Intelligence Enhanced Digital Nucleic Acid Amplification Testing for Precision Medicine and Molecular Diagnostics. 2024. DOI: 10.48550/arxiv.2407.21080
[11] Digital PCR as an emerging tool for monitoring of microbial biodegradation. Molecules. 2020. DOI: 10.3390/MOLECULES25030706
[12] Weidner, C., Frenzel, J., Bartsch, D., et al. Guideline for the verification of digital PCR methods in analytical GMO testing. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2024. DOI: 10.1007/s00003-024-01516-6
[13] A single-cell digital PCR method tailored for quantification of HBV DNA positive cells in liver biopsy tissues. Journal of Translational Medicine. 2025. DOI: 10.1186/s12967-025-07131-9
[14] Manivannan, R., Tamilselvan, C., Gopal, R.K., et al. Microbial Diseases of Laboratory Animals and its monitoring Tools. Journal of Advances in Microbiology. 2024. DOI: 10.9734/jamb/2024/v24i2794
[15] Clinical utility impact of DNA-based cytology using droplet digital methylation-specific PCR in gastric cancer. Gastric Cancer. 2025. DOI: 10.1007/s10120-025-01674-y
Recent Posts
-
The Role of Technetium-99m in Sentinel Lymph Node Detection for Gynecological Cancers
The Role of Technetium-99m in Sentinel Lymph Node Detection for Gynecological Cancers Int …3rd Nov 2025 -
Unlocking the Secrets of Genetic Variation: A Deep Dive into SDR-seq
Unraveling the Impact of Coding and Noncoding Variants on Gene Expression Summary Scientists at the …21st Oct 2025 -
Current Bioinformatics Tools in Precision Oncology: An Integrated Review for Researchers
Current Bioinformatics Tools in Precision Oncology Abstract Precision oncology has transitioned from …14th Oct 2025