Current Bioinformatics Tools in Precision Oncology: An Integrated Review for Researchers
Current Bioinformatics Tools in Precision Oncology
Abstract
Precision oncology has transitioned from conventional, one-size-fits-all treatments to personalized strategies driven by high-throughput multiomics and bioinformatics. This review synthesizes the latest bioinformatics tools and computational frameworks that enable the discovery and validation of cancer biomarkers. Covering genomics, transcriptomics, proteomics, epigenomics, and metabolomics, it explores how artificial intelligence (AI) and machine learning (ML) are redefining the predictive and diagnostic landscape in oncology. The integration of these technologies supports biomarker-driven therapy design, predictive modeling, and patient stratification, establishing bioinformatics as the cornerstone of modern cancer research.
1. Introduction
The paradigm shift toward precision oncology is fueled by advances in sequencing and computational biology that enable individualized cancer care. Bioinformatics at the intersection of biology, mathematics, and computer science has become indispensable for processing and interpreting massive datasets generated by next-generation sequencing (NGS), transcriptomics, and proteomics. By identifying and validating molecular biomarkers such as mutations, copy number variations, and epigenetic signatures, bioinformatics facilitates the development of patient-specific treatment strategies.
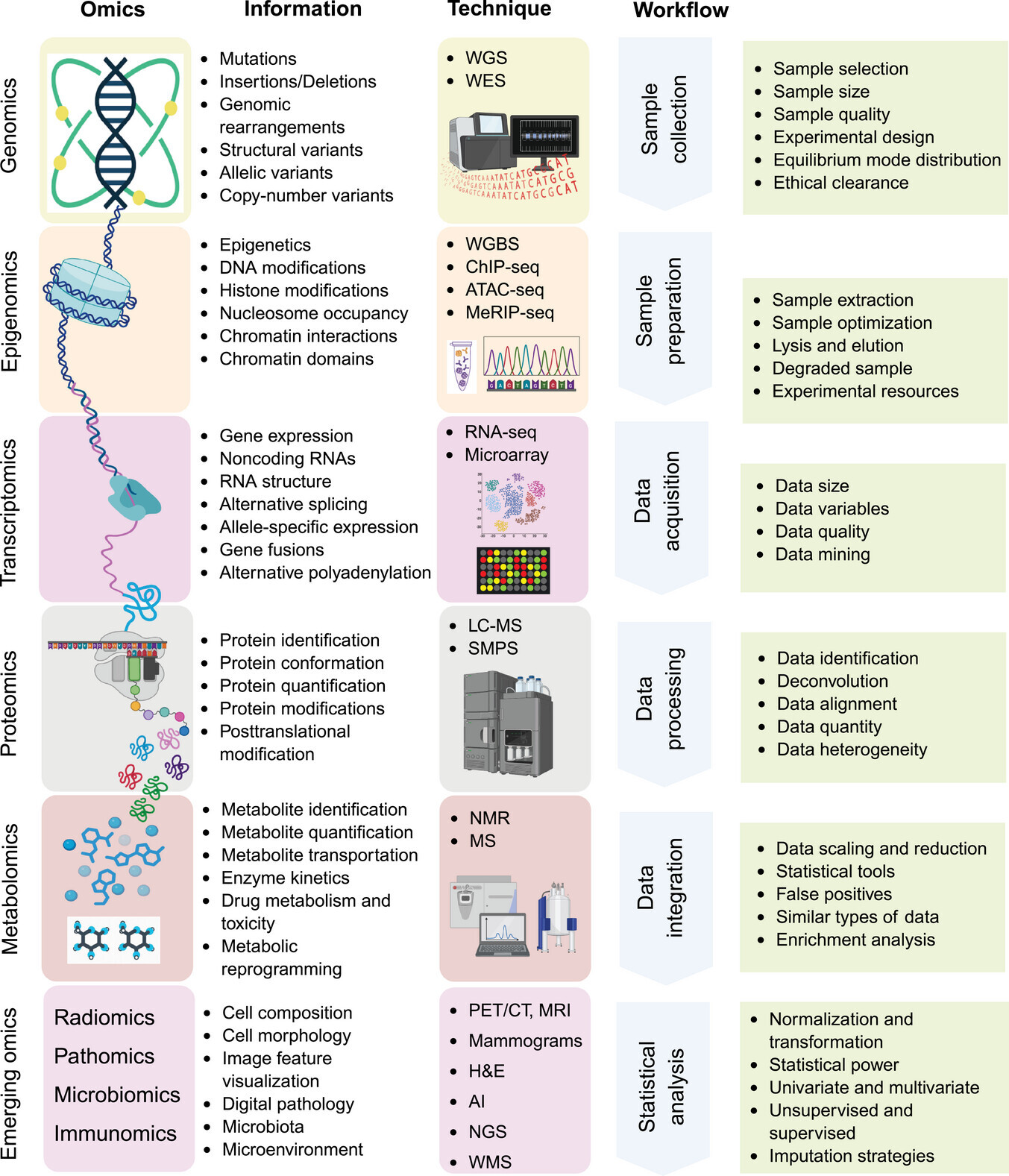
Types of Omics Data in Biomarker Discovery
The identification and validation of biomarkers in precision oncology are largely driven by omics technologies, which enable the large-scale characterization of biological molecules across multiple levels of cellular function.
The term “omics” encompasses a range of disciplines genomics, transcriptomics, proteomics, epigenomics, and metabolomics each providing a unique yet complementary perspective on cancer biology.
-
Genomics reveals DNA-level alterations such as mutations and copy number variations.
-
Transcriptomics examines RNA expression to uncover gene regulation dynamics.
-
Proteomics investigates protein abundance and post-translational modifications.
-
Epigenomics explores heritable regulatory mechanisms like DNA methylation and histone modification.
-
Metabolomics profiles cellular metabolites that reflect physiological and pathological states.
2. Genomics: The Foundation of Precision Oncology
Genomics forms the basis of biomarker discovery by decoding the mutational landscape of cancer. High-throughput sequencing techniques, including whole-genome sequencing (WGS), whole-exome sequencing (WES), and single-cell DNA sequencing, allow the identification of single-nucleotide polymorphisms (SNPs), insertions/deletions (indels), and copy number variations (CNVs) associated with tumorigenesis.
Core bioinformatics tools:
-
GATK and MuTect2 for variant calling and somatic mutation detection
-
ANNOVAR for functional annotation
-
CellRanger, Monocle 3, and Morpheus for single-cell genomic profiling and lineage tracing
These tools enable researchers to translate raw sequencing data into actionable clinical insights, illuminating the genetic heterogeneity that drives cancer development and therapy resistance.
3. Transcriptomics: Mapping Gene Expression Dynamics
Transcriptomics captures gene expression changes that reflect tumor behavior. RNA sequencing (RNA-seq) and single-cell transcriptomics reveal regulatory patterns underlying oncogenic transformation.
Key computational platforms:
-
DESeq2, EdgeR, and limma statistical packages for differential gene expression analysis
-
Salmon and Kallisto alignment-free quantification of transcripts
-
Seurat, Scanpy, and CellBender clustering, noise correction, and single-cell trajectory modeling
-
cBioPortal and pyBioPortal integrate transcriptomic data with clinical outcomes
These tools elucidate expression signatures that differentiate tumor subtypes, predict drug response, and guide prognostic assessments.
4. Proteomics: From Sequence to Function
Proteomics explores the functional output of the genome by identifying and quantifying proteins and post-translational modifications (PTMs). Mass spectrometry (MS) and liquid chromatography–MS/MS (LC–MS/MS) are the principal techniques in high-throughput proteome analysis.
Notable tools:
-
MaxQuant, Skyline, Proteome Discoverer, OpenMS, and PeptideShaker for protein identification and quantification
-
STRING, Cytoscape, and BioGRID for protein–protein interaction mapping
-
PhosphoSitePlus and AlphaFold for PTM analysis and structural prediction
Proteomic data complement genomic and transcriptomic findings, revealing the molecular mechanisms underlying tumor phenotype and therapeutic susceptibility.
5. Epigenomics: Decoding the Regulatory Layer
Epigenetic alterations DNA methylation, histone modification, and noncoding RNA interactions play crucial roles in oncogenesis. Bioinformatics-driven epigenomic profiling identifies reversible molecular markers that can serve as diagnostic and therapeutic targets.
Principal tools:
-
Bismark, MethylKit, and DSS for methylation analysis
-
MACS and SICER for ChIP-seq and ATAC-seq peak detection
-
ChromHMM and DiffReps for chromatin state modeling
-
EpiDISH and BSmooth for regulatory network deconvolution
Epigenomics bridges gene regulation with phenotype, offering novel therapeutic entry points such as epigenetic inhibitors and methylation-based diagnostics.
6. Metabolomics: Capturing the Biochemical Fingerprint
Metabolomics focuses on the end products of cellular metabolism, capturing metabolic shifts characteristic of cancer such as the Warburg effect. These signatures aid in early detection and therapeutic monitoring.
Analytical and computational tools:
-
XCMS, MZmine, and OpenMS for LC–MS and GC–MS data analysis
-
MetaboAnalyst, MetFrag, and GNPS for metabolite identification and pathway enrichment
-
KEGG, HMDB, and Reactome for metabolic network integration
By linking metabolic phenotypes with genetic and proteomic profiles, metabolomics enables a holistic understanding of tumor metabolism.
7. Multiomics Integration and Biomarker Validation
Integrative bioinformatics merges multi-layered omics data to uncover comprehensive biomarker signatures. Platforms such as iCluster, MixOmics, MOFA+, and Sangerbox 3 combine genomic, proteomic, and metabolomic profiles for robust biomarker identification.
Validation frameworks:
-
TCGA, GEO, and ICGC for in silico and cross-cohort validation
-
SurvExpress, GEPIA, and TIMER for prognostic evaluation and immune correlation
-
Cox proportional hazard models for survival association analyses
This stepwise validation ensures biomarker reproducibility and clinical translatability, bridging computational discovery with patient outcomes.
8. Artificial Intelligence and Machine Learning in Oncology
AI and ML have redefined biomarker discovery by enabling predictive modeling across massive, multidimensional datasets.
Supervised methods (e.g., Random Forests, SVMs) and unsupervised methods (e.g., PCA, clustering) facilitate classification, while deep learning architectures (CNNs, RNNs) extract complex patterns from histopathological and sequencing data.
Prominent AI frameworks:
-
TensorFlow, PyTorch, scikit-learn, XGBoost, and Keras for predictive analytics
-
DeepVariant and PandOmics for AI-enhanced variant and biomarker detection
-
Explainable AI (XAI) tools like SHAP and LIME for model transparency
By integrating AI-driven analytics with clinical and multiomics data, researchers can predict drug sensitivity, identify therapeutic targets, and stratify patients with unprecedented precision.
9. Challenges and Future Directions
Despite transformative progress, challenges persist: data heterogeneity, batch effects, limited interoperability, and the need for robust clinical translation. The future of precision oncology will rely on:
-
Cloud-based data harmonization platforms (e.g., DNAnexus, Galaxy)
-
Federated learning for privacy-preserving multi-institutional collaboration
-
Spatial omics integration linking molecular signatures with tumor microenvironments
-
AI-assisted model interpretability to build clinician trust
The convergence of AI, high-performance computing, and multiomics integration will continue to shape the next era of biomarker-driven cancer therapeutics.
Conclusion
Bioinformatics tools are the backbone of precision oncology, enabling researchers to navigate the vast landscape of genomic and molecular data. From sequencing to AI-driven prediction, these computational frameworks are transforming cancer diagnostics, prognostics, and therapeutics. The ongoing fusion of multiomics with machine learning not only accelerates biomarker discovery but also heralds a future where precision oncology becomes the universal standard of cancer care.
Recent Posts
-
The Role of Technetium-99m in Sentinel Lymph Node Detection for Gynecological Cancers
The Role of Technetium-99m in Sentinel Lymph Node Detection for Gynecological Cancers Int …3rd Nov 2025 -
Unlocking the Secrets of Genetic Variation: A Deep Dive into SDR-seq
Unraveling the Impact of Coding and Noncoding Variants on Gene Expression Summary Scientists at the …21st Oct 2025 -
Current Bioinformatics Tools in Precision Oncology: An Integrated Review for Researchers
Current Bioinformatics Tools in Precision Oncology Abstract Precision oncology has transitioned from …14th Oct 2025